Evoque Tricuspid Valve Replacement System
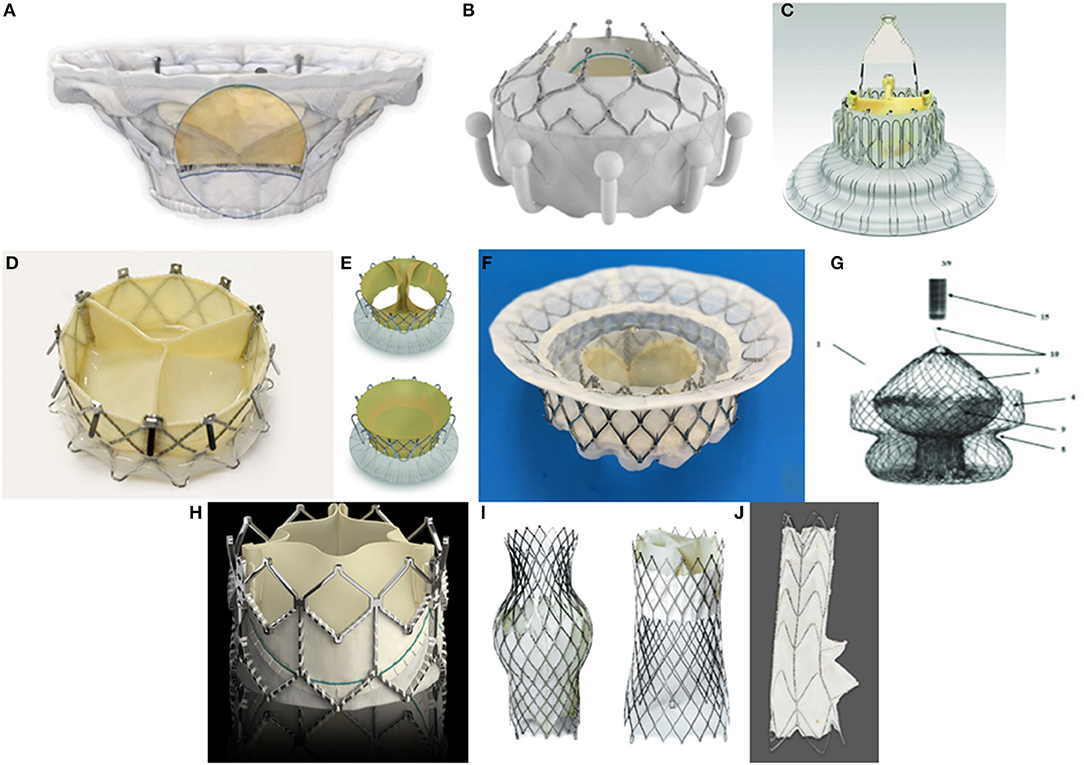
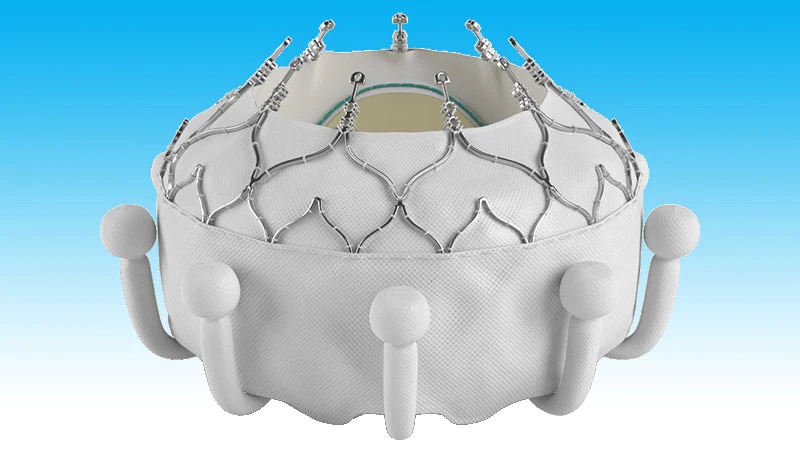
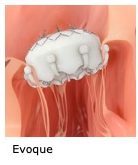
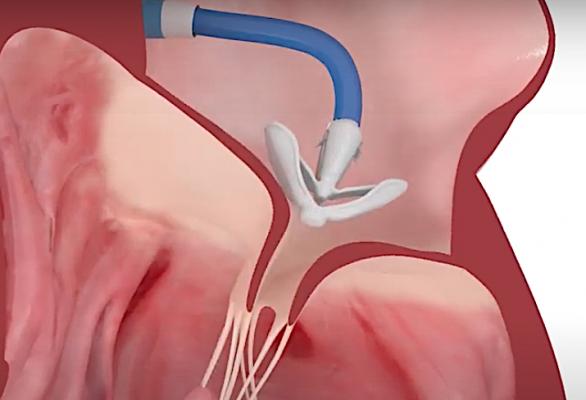
Evoque tricuspid valve replacement system. Six-month results from the TRISCEND study of the Evoque Edwards Lifesciences transcatheter tricuspid valve replacement system were presented during a late-breaking trial session at the Transcatheter Cardiovascular Therapeutics annual meeting TCT 2021 46 November Orlando USA and virtual. The Evoque tricuspid valve replacement system Edwards Lifescience Irvine CA USA Figure 1B resembles its mitral valve counterpart comprised of bovine pericardial leaflets with an intra-annular sealing skirt and anchors Table 1. IRVINE Calif Nov.
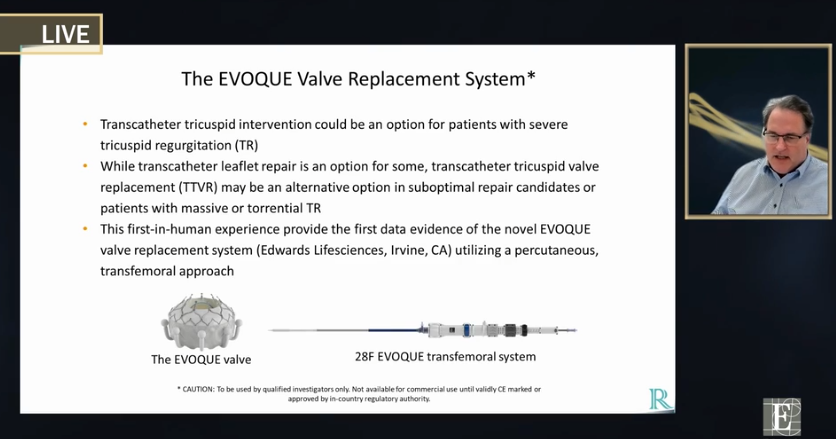
Edwards Evoque system. Tricuspid valve replacement TTVR may be preferred in suboptimal repair candidates or patients with massive or torrential TR This first-in-human experience assessed the feasibility and safety of the novel EVOQUE valve replacement system Edwards Lifesciences Irvine CA utilizing a percutaneous transfemoral approach Neil Fam. EW announced that results from a clinical trial of the companys EVOQUE transcatheter tricuspid valve replacement system demonstrated that favorable patient outcomes were sustained at six months.
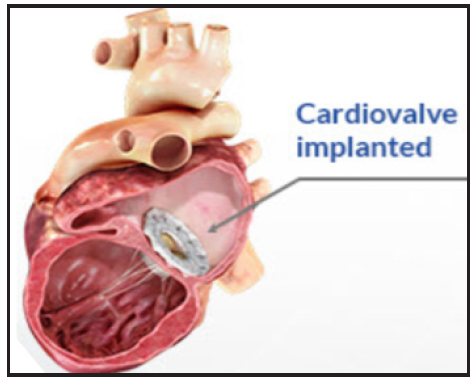
In collaboration with cardiologists like you Edwards transcatheter mitral and tricuspid therapies are making a meaningful mark on the lives of patients with mitral and tricuspid regurgitation. The valve is implanted using a minimally invasive delivery system where your doctor places a thin tube through a vein in your leg to reach your heart and perform the valve replacement. A Multicenter Observational First-in-Human Experience JACC Cardiovasc Interv.
The investigational transfemoral EVOQUE tricuspid valve replacement system Edwards Lifesciences significantly reduced severity of tricuspid regurgitation and. The team used the EVOQUE tricuspid valve replacement system Edwards Lifesciences Irvine CA. EW announced that results from a clinical trial of the companys EVOQUE transcatheter tricuspid valve replacement system demonstrated that favorable.
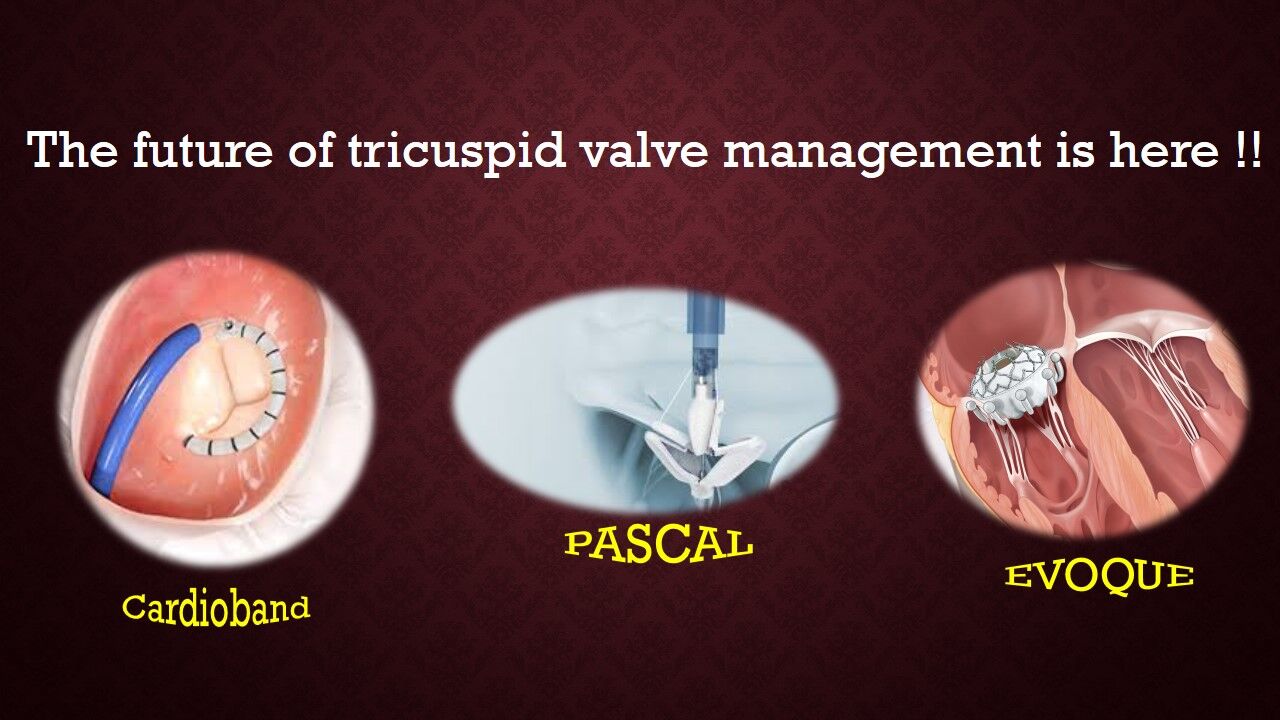
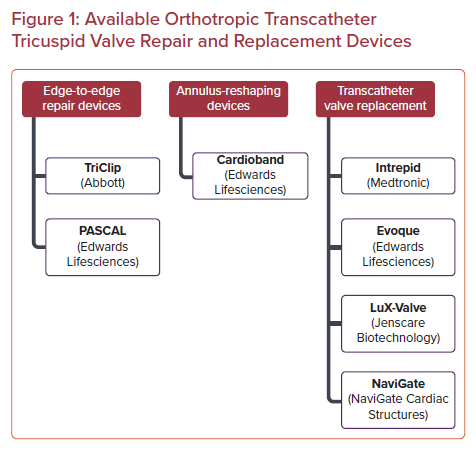
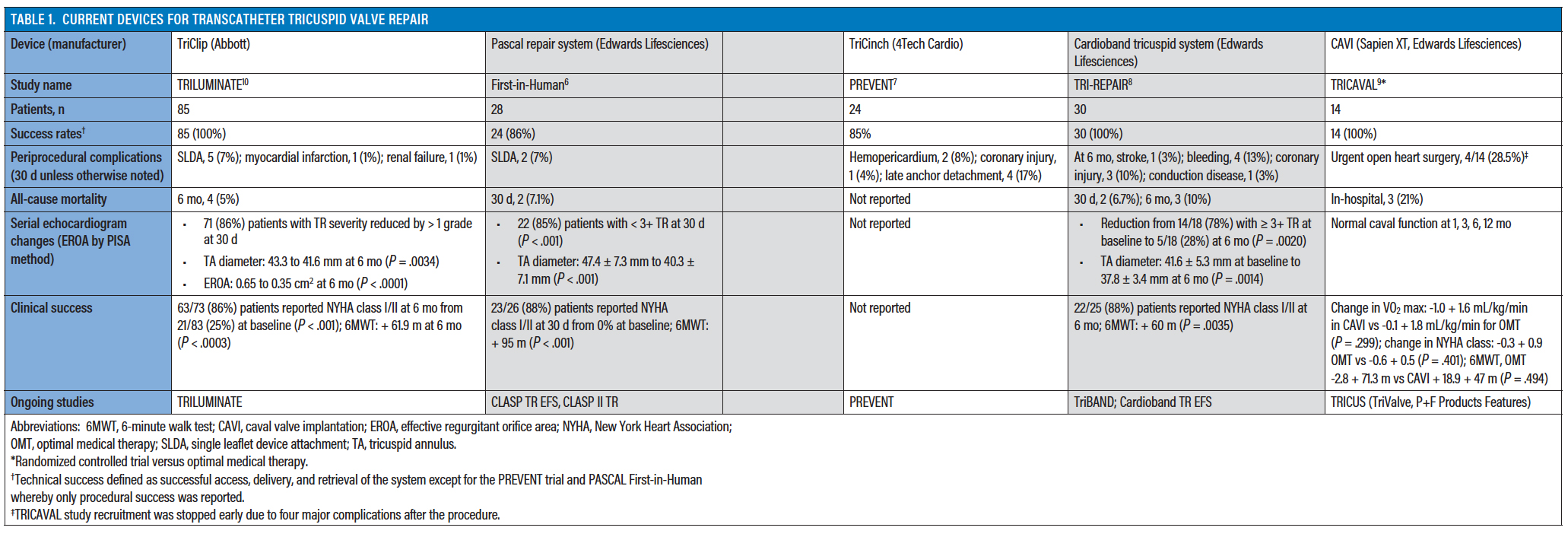
What is the EVOQUE tricuspid valve replacement system. Transcatheter tricuspid valve replacement with the Edwards EVOQUE System in conjunction with optimal medical therapy OMT in patients with tricuspid regurgitation who are not eligible for randomization. The TriClip system is designed to reduce the amount of tricuspid regurgitation without the need for surgery less invasive.
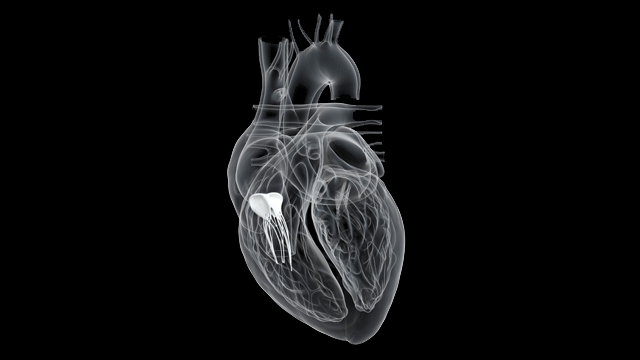
The Edwards EVOQUE tricuspid valve replacement system is an investigational device designed to replace your native tricuspid valve without open-heart surgery. The Evoque Device - So the Evoque is a transcatheter tricuspid valve replacement. And the EVOQUE system from Edwards is designed to replace the tricuspid valve in a native tricuspid annulus via a transfemoral percutaneous approach.
EW announced that results from a clinical trial of the companys EVOQUE transcatheter tricuspid valve replacement system demonstrated that favorable patient outcomes were sustained at six months. Results from the TRISCEND study treating patients with tricuspid.
6 2021 PRNewswire -- Edwards Lifesciences Corporation NYSE.
EW announced that results from a clinical trial of the companys EVOQUE transcatheter tricuspid valve replacement system demonstrated that favorable. EW announced that results from a clinical trial of the companys EVOQUE transcatheter tricuspid valve replacement system demonstrated that favorable patient outcomes were sustained at six months. The TRISCEND study is a prospective single-arm multicenter study designed to evaluate the safety and performance of the transfemoral EVOQUE tricuspid valve replacement system in TR. Edwards Evoque system. TRISCEND investigator Susheel Kodali Columbia. The 28-F EVOQUE valve replacement system for TR which is identical to the system for mitral regurgitation is then introduced over the wire and advanced to the tricuspid valve. The investigational transfemoral EVOQUE tricuspid valve replacement system Edwards Lifesciences significantly reduced severity of tricuspid regurgitation and. The team used the EVOQUE tricuspid valve replacement system Edwards Lifesciences Irvine CA. The Evoque tricuspid valve replacement system Edwards Lifescience Irvine CA USA Figure 1B resembles its mitral valve counterpart comprised of bovine pericardial leaflets with an intra-annular sealing skirt and anchors Table 1.
The EVOQUE valve replacement system is an investigational device and is not available for sale in any country. The Evoque Device - So the Evoque is a transcatheter tricuspid valve replacement. 6 2021 PRNewswire -- Edwards Lifesciences Corporation NYSE. The TRISCEND study is a prospective single-arm multicenter study designed to evaluate the safety and performance of the transfemoral EVOQUE tricuspid valve replacement system in TR. Edwards Evoque valve is used to replace a patients tricuspid valve and is administered through a transcatheter procedure by going through the femoral artery in a patients thigh. The Edwards EVOQUE tricuspid valve replacement system is an investigational device designed to replace your native tricuspid valve without open-heart surgery. What was the design patient population and endpoints.
































Post a Comment for "Evoque Tricuspid Valve Replacement System"